COVID-19 IgG/IgM Rapid Test at wholesale
Negotiated Price
Dear sir / madam,
We update the latest price of 《COVID-19IgG/IgM Rapid Test 》 as follows:
10000-100000 (US $4.5, same day delivery)
100000-500000 (US $4.2, delivery within 2 days)
500000-1000000 (US $3.9, delivery within 3 days)
1000000-5000000 (US $3.8, 4-10 days delivery)
Over 5000000 (US $3.7, delivery within 20 days)
The above prices are FOB
Payment method: T / T
Payment: The buyer will pay 50% through TT as a down payment before the seller starts the production. After all the products finish, the buyer pay the left balance 50% by TT.
In order to avoid unnecessary misunderstanding, I need to tell you some things in advance: COVID - 19 outbreak is about half a year now, and in view of the needed inmanufacturing COVID - 19 of the CE and FDA certificate, the normal application process can take one to two years, all that is in the market now claims to have the CE and FDA certificate are all false, I hope you will seriously to this problem. However, in order to prevent the spread of the epidemic and expand the number of people tested, CE standard has introduced a declaration of compliance, and FDA standard has introduced the emergency standard EUA. This declaration and emergency standard can now be recognized as having the same effect as CE and FDA. Among the materials I sent to you, one is the CE compliance statement, and the other is the application record of EUA. Now our manufacturers are monitoring and collecting clinical data in the United States, so as to get the FDA certificate as soon as possible. Therefore, there is no doubt about the strength of our manufacturers, otherwise our manufacturers would not have the courage and confidence to spend a lot of time and energy to get the certificate.
At the same time, our manufacturer is also on the white list of test reagent export of the ministry of commerce of the Chinese government. If you need to inquire, I can also provide you with screenshots of relevant procedures.
《We can provide you with formal sales authorization separately》
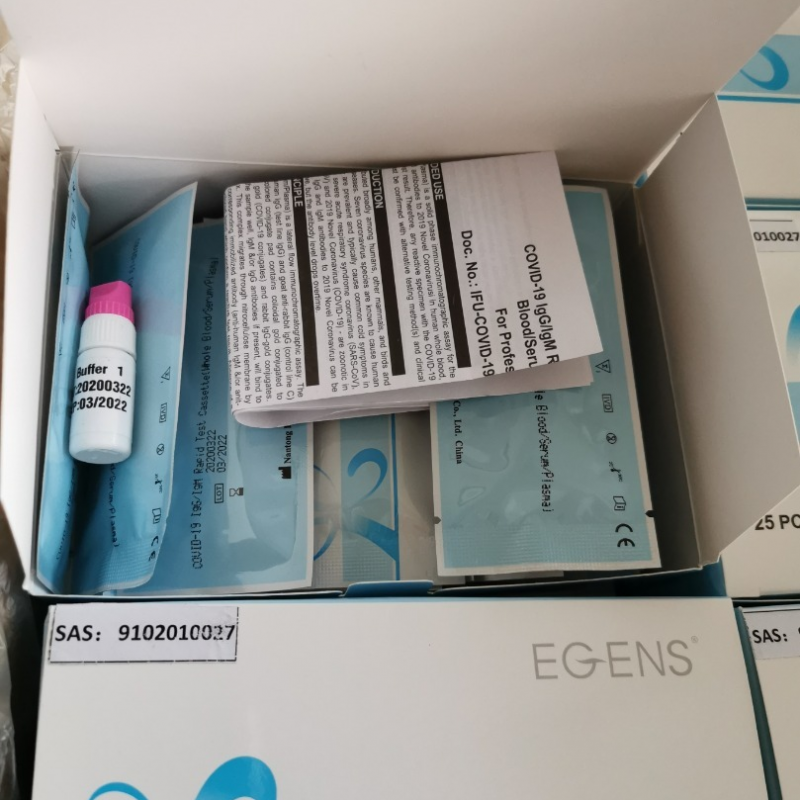
There is an important message I need to convey to you. Our test kit can now be individually formulated according to each person ’s diluent, which is equivalent to each person ’s kit having an independent diluent. Previously, 25 people shared a bottle of diluent Now it can be produced separately according to customer needs,You can now directly face the market for wholesale and retail, the practical value of the product will further increase.
Now making money is not the first, saving lives is the first. We need to work with long-term trustworthy buyers to help more people who really need help.





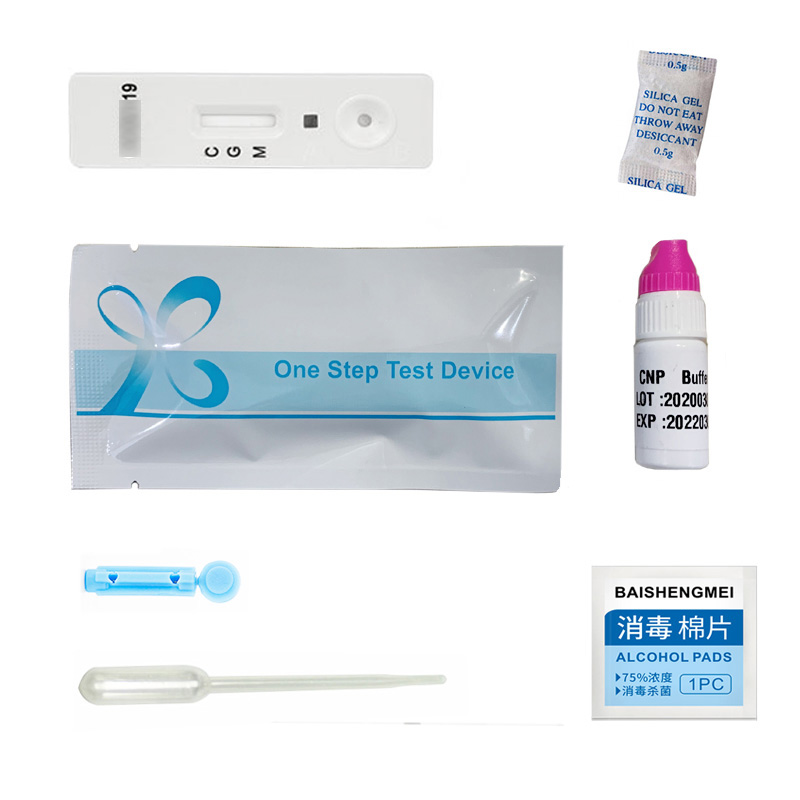

 China
China